The creators, John B. Goodenough, Akira Yoshino, and M. Stanley Wittingham, were responsible for the creation of lithium-ion batteries. The main power source for electric cars. This amazing development was the reason the Nobel Prize was awarded to the trio.
This new source of energy feeds not only electric cars, but also some mobile devices such as smartphones, laptops, and more.
Even though it is already establishing itself on the market, the battery cells took a long time to study and develop before the release for commercialization. In an official announcement, we can see that the studies started quite some time ago, in the 1970s:
“They created a rechargeable world
The Nobel Prize in Chemistry 2019 rewards the development of lithium-ion batteries. This lightweight, rechargeable, and a powerful battery is now used in everything from mobile phones to laptops and electric vehicles. It can also store significant amounts of energy from solar and wind power, making possible a fossil fuel-free society.
Lithium-ion batteries are used globally to power the portable electronics that we use to communicate, work, study, listen to music, and search for knowledge. Lithium-ion batteries have also enabled the development of long-range electric cars and the storage of energy from renewable sources, such as solar and wind power.
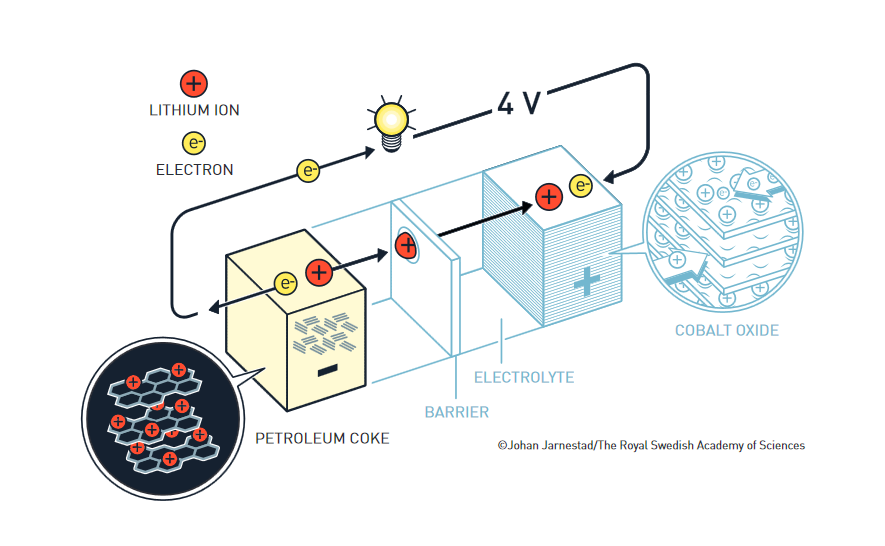
The foundation of the lithium-ion battery was laid during the oil crisis in the 1970s. Stanley Whittingham worked on developing methods that could lead to fossil-fuel-free energy technologies. He started to research superconductors and discovered an extremely energy-rich material, which he used to create an innovative cathode in a lithium battery. This was made from titanium disulphide which, at a molecular level, has spaces that can house – intercalate – lithium ions.
The battery’s anode was partially made from metallic lithium, which has a strong drive to release electrons. This resulted in a battery that literally had great potential, just over two volts. However, metallic lithium is reactive, and the battery was too explosive to be viable.
John Goodenough predicted that the cathode would have even more significant potential if it were made using a metal oxide instead of metal sulfide. After a systematic search, in 1980, he demonstrated that cobalt oxide with intercalated lithium ions can produce as much as four volts. This was a significant breakthrough and would lead to much more powerful batteries.“
Each one of the winners will also receive a financial award of $900,000.