For years, supercapacitors have been on the back burner as an alternative to lithium batteries in energy storage. A system with great potential whose main difference compared to lithium batteries is that they are physical, not chemical, accumulators, and their storage system is based on the separation of charges. Charges move much faster than lithium ions (lithium ions, for example) and can offer much more power and charge in just seconds.
The main problem with this technology is its low energy density, between 20 and 30 times less than current lithium batteries. But this does not stop the investigations that seeks to commercially exploit a technology that will offer enormous advantages, such as its excellent power transmission capacity, which will allow them to provide alternatives with high needs and compatibility with ultra-fast recharges. Ideal to meet the demands of large vehicles.
Now the University of Texas has presented the first results of some good works describing a novel energy storage device based on organic materials, which will also have characteristics such as being flexible, light, and very economical when using conventional and inexpensive components.
Supercapacitors have an internal architecture similar to that of essential capacitors. Both devices store the charge on metal plates or electrodes. However, unlike capacitors, supercapacitors can be manufactured in different sizes, shapes, and designs, depending on the intended application. Furthermore, supercapacitor electrodes can also be constructed from other materials.
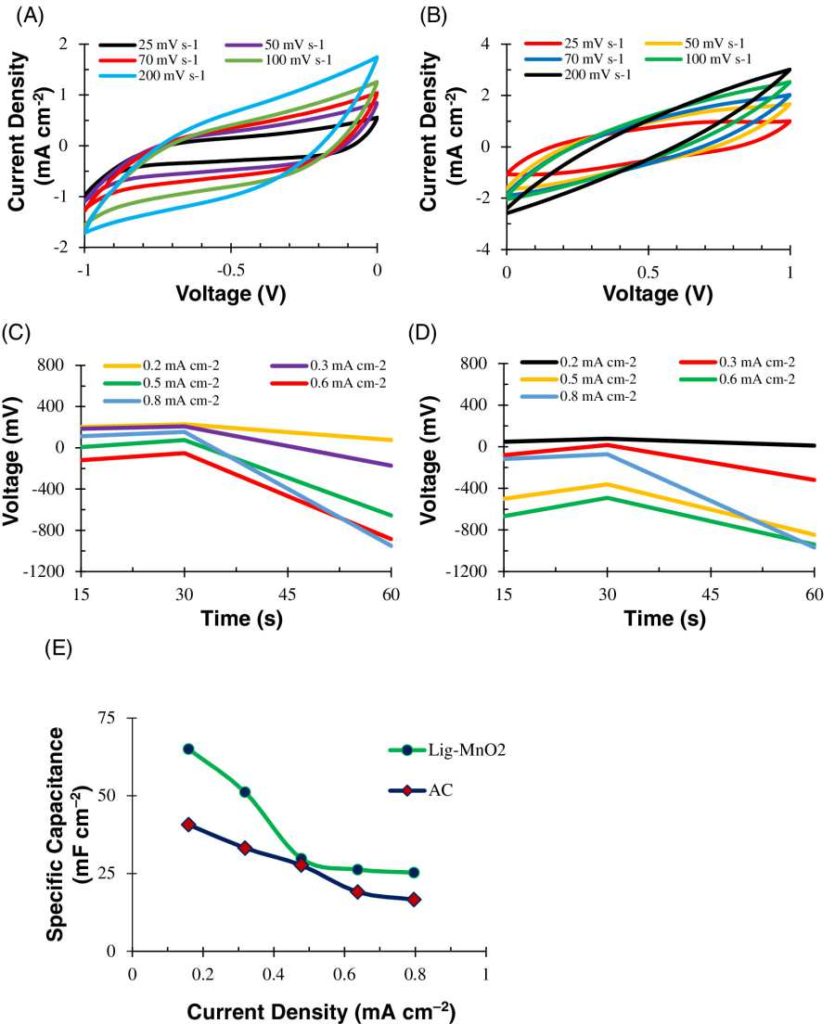
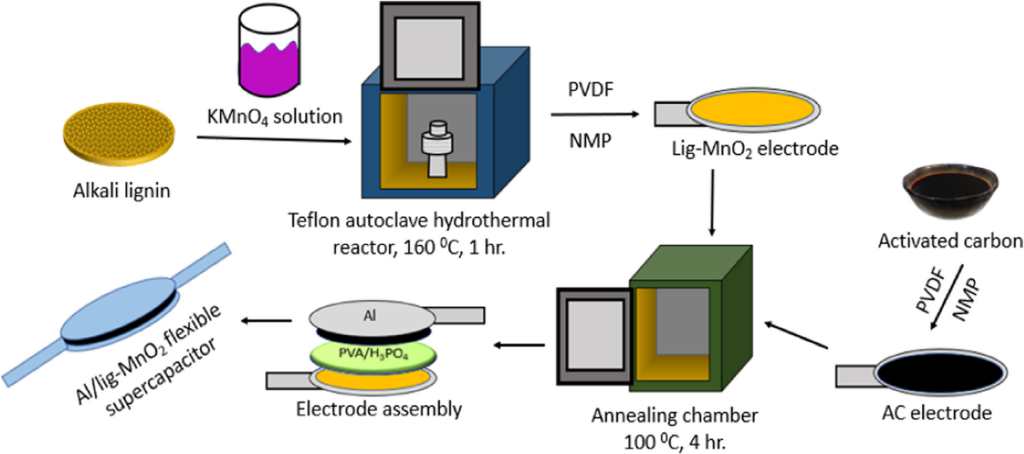
For this project, those responsible have chosen to design one of the two electrodes, a compound formed by nanoparticles of manganese dioxide. The university team has explained that manganese dioxide is cheaper and is available in abundance and safer than other oxides, such as ruthenium or zinc oxide, which are popularly used for manufacturing electrodes.
The significant disadvantage of manganese dioxide is that it has low electrical conductivity. To solve this problem, the team used lignin, an organic polymer, purified with potassium permanganate. They then applied high pressure and heat to start an oxidation reaction that results in the decomposition of potassium permanganate and the deposition of manganese dioxide in lignin. Next, they coated the mixture of lignin and manganese dioxide on an aluminum plate to form the green electrode. Finally, the researchers assembled the supercapacitor by inserting a gel electrolyte between the lignin-manganese dioxide-aluminum electrode and another electrode made of aluminum and activated carbon.
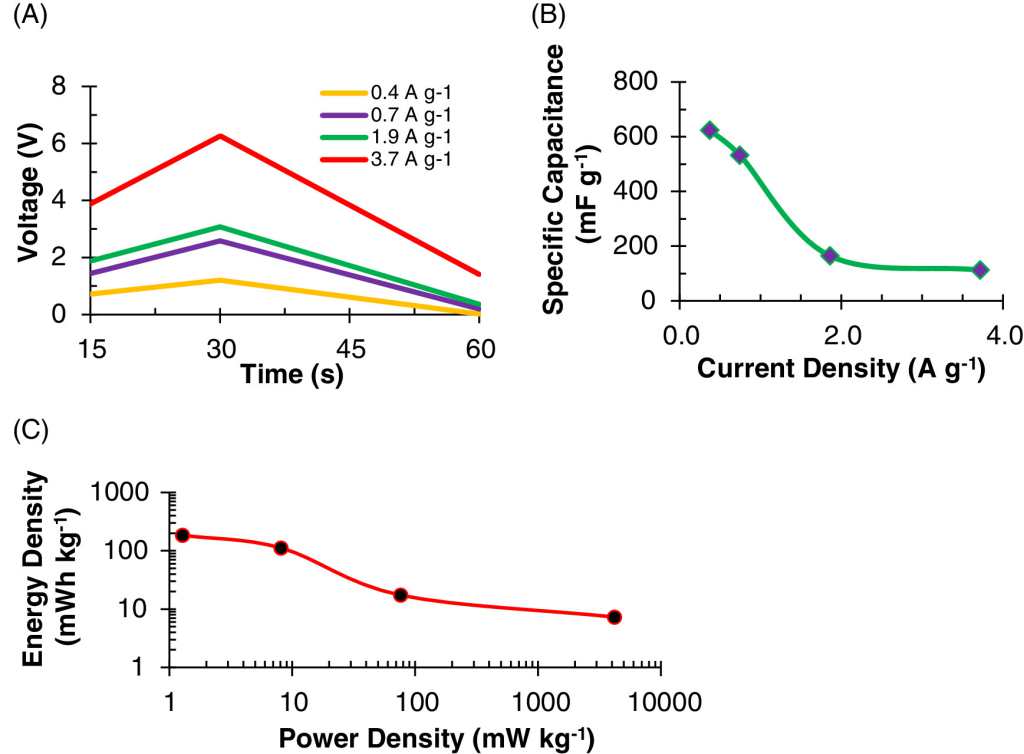
The result of this compound has been a supercapacitor with very stable electrochemical properties. In particular, the specific capacitance, or the device’s ability to store an electrical charge, changed little even after thousands of charge and discharge cycles. Also, for an optimal lignin-manganese dioxide ratio, the specific capacitance was found to be up to 900 times higher than what has been reported for other supercapacitors.
Also, it has been indicated that the result is a type of light and flexible accumulators. Something that opens the doors to the possibilities of being installed even in the vehicles’ structure and adapting to existing platforms would reduce their development.